- Facilitates patient treatment decisions quickly
- High sensitivity and specificity
- No instrumentation required
- Simple, time-saving procedure
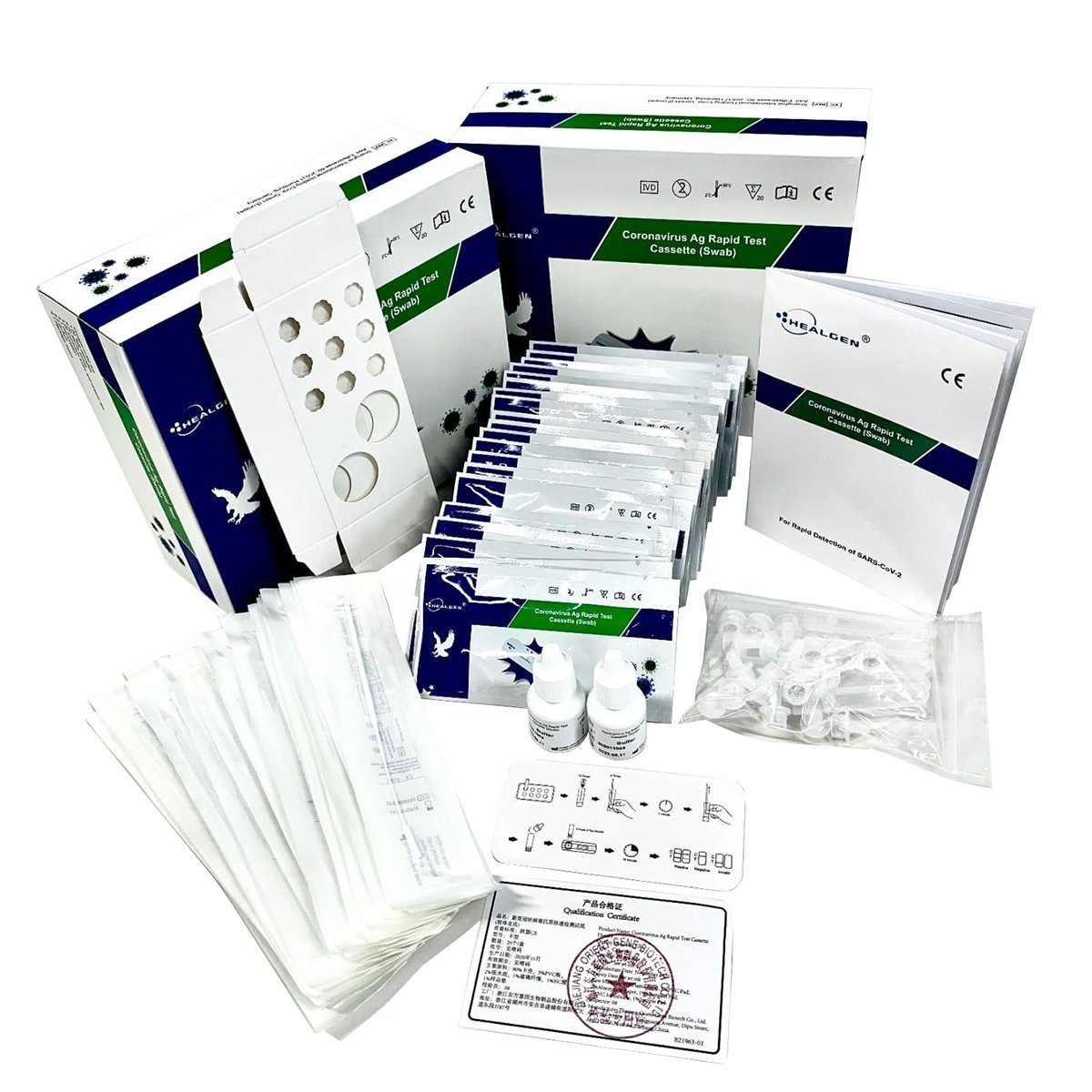
The Coronavirus Ag Rapid Test Cassette (Swab) is an in vitro immunochromatographic assay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) swab specimens directly from individuals who are suspected of COVID-19 by their healthcare provider within the first 10 days of symptom onset. It is intended to aid in the rapid diagnosis of SARS-CoV02 infections.
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. Currently, individuals infected by SARS-CoV-2 are the main source of transmission; asymptomatic carriers are also known as a source of transmission. Based on the current epidemiological information, the incubation period can last from 1 to 14 days, where the average time before symptoms occur is about 5 days. Symptoms include fever or chills, cough, shortness of breath, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, runny nose, nausea or vomiting and diarrhea
Warning: The Coronavirus Ag Rapid Test Cassette (swab) does not differentiate between SARS-CoV and SARS-CoV-2. This test is not for home-use or at-home specimen collection.
What is antigen testing?
Antigen tests detect proteins specific to a virus that appears in infected individuals, which indicates active infection. Rapid antigen testing is beneficial because of their portability, ease-of-use, and swift time to result.
Who can carry out antigen testing?
It is recommended that tests be carried out by clinical professionals such as doctors, practice nurses, employer occupational health departments or trusted carers or colleagues.
What is the extraction buffer?
The extraction buffer includes components to dissociate viral proteins from their surfaces to be used as a target for the test. The method of the extraction buffer is used to prepare the test sample by dissolving the virus shell, which also deactivates the virus.
Does the test kit include transport media?
The test kit includes an extraction buffer that is used to prepare the specimen for testing purposes. The extraction buffer IS NOT transport media and should not be used to preserve or transport specimens.
Can the test be used with alternative specimen types such as nasal swabs or any specimen contained in viral transport media?
The Rapid COVID-19 Antigen Test is for direct nasopharyngeal swab specimen only. The test can only be used with the swab provided in the kit.
Can I collect the specimen and test at a later time?
The specimen should be tested immediately after collection. The specimen can be retained up to 1 hour following collection if immediate testing of specimens is not possible. Dispose of the specimen and recollect if retained for more than 1 hour.
Can I freeze the device for long-term storage?
The device should never be frozen. If refrigerated, allow the test kit components to reach room temperature before use.
How do I dispose of used tests?
It's recommended that used tests are disposed of in medical waste bins.